Mass of One Atom of Carbon 12
The atomic mass or atomic weight is the decimal number The number of significant figures varies according to the table but the value is around 1201. Because a dalton a unit commonly used to measure atomic mass is exactly 112 of the mass of a carbon-12 atom this definition of the mole entailed that the mass of one mole of a compound or element in grams was numerically equal to the average mass of one molecule or atom of the substance in daltons and that the number of daltons in a gram was equal to the number of.

How To Find The Mass Of One Atom Of Carbon C Youtube
Molar mass of C 120107 gmol.

. Discussion Forum Carbon molecular weight. Atomic mass the quantity of matter contained in an atom of an element. The atomic mass m a or m is the mass of an atomAlthough the SI unit of mass is the kilogram symbol.
Therefore C-atom in CF 3 I is more electronegative than in CH 3 I. This is incredibly easy with isotopes as they are named according to their atomic mass. There is a gravitational force between any two masses but it is very small except when one or both of the objects have large.
In water there are 2 Hydrogen atoms and 1 Oxygen atom. One atomic mass unit u is equal to 112 the mass of one atom of carbon-12. Calculate the molar mass of Carbon in grams per mole or search for a chemical formula or substance.
Each atom has a charged substructure consisting of a nucleus which is made of protons and neutrons surrounded by electrons. In other words a relative atomic mass tells you the number of times an average atom of an element from a given sample is heavier than one-twelfth of an atom of. Find the atomic mass.
Atomic mass of Carbon 1201. Kg atomic mass is often expressed in the non-SI unit dalton symbol. The electronegativity of an atom depends upon the nature of the substituent attached to that atom.
The protons and neutrons of the nucleus account for nearly all of the. Da equivalently unified atomic mass unit u. This is not the value you want.
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of. We can find the atomic weight of hydrogen and oxygen from the periodic table. Atomic mass of Oxygen 1600.
It is also sometimes called. Once you find the atomic mass of the isotope the process is the same as it is for finding the number of neutrons in a regular atom. Molar mass is the mass in atomic mass units of one mole of a of a substance.
We can also use molecular weight calculator for finding molar. In this scale 1 atomic mass unit amu corresponds to 1660539040 1024 gram. 1 Da is defined as 1 12 of the mass of a free carbon-12 atom at rest in its ground state.
Carbon-14 for example has an atomic mass of 14. Molecular Mass Molecular Weight Formula Mass or Formula Weight. The difference in electronegativity of an atom caused by substituents results in different.
It is the ratio of the average mass per atom of an element from a given sample to 112 the mass of a carbon-12 atom. One mole of carbon is 6022 x 10 23 atoms of carbon Avogadros number. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom 1992646547 1023 gram which is assigned an atomic mass of 12 units.
This number 1201 is the mass in grams of one mole of carbon. This relation is then used to convert a carbon atom to grams by the ratio. For example the carbon atom in CF 3 I acquires a greater positive charge than CH 3 I.
A r is a measure of how heavy atoms are. How can I find the molar mass of an element. To calculate the mass of a single atom first look up the atomic mass of carbon from the periodic table.
The molar mass of elements is found by looking at. This way we can calculate the molar mass of a compound or one-carbon compound. By the end of grade 12.
The atomic mass unit is also called the dalton Da after English. An atomic mass unit symbolized AMU or amu is defined as precisely 112 the mass of an atom of carbon-12. One number is carbons element number or atomic number.
The carbon-12 C-12 atom has six protons and six neutrons in its nucleus. A relative atomic mass also called atomic weight. Atomic number increase as you go across the table.
Eg synthesis of sugars from carbon dioxide and water. Atomic mass unit AMU or amu.
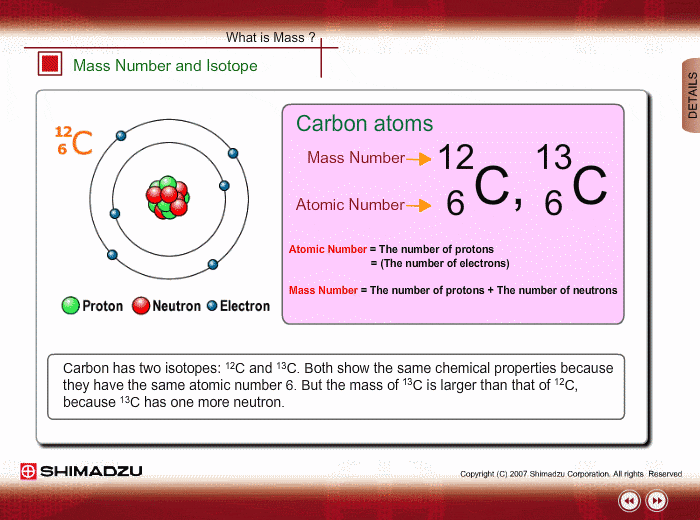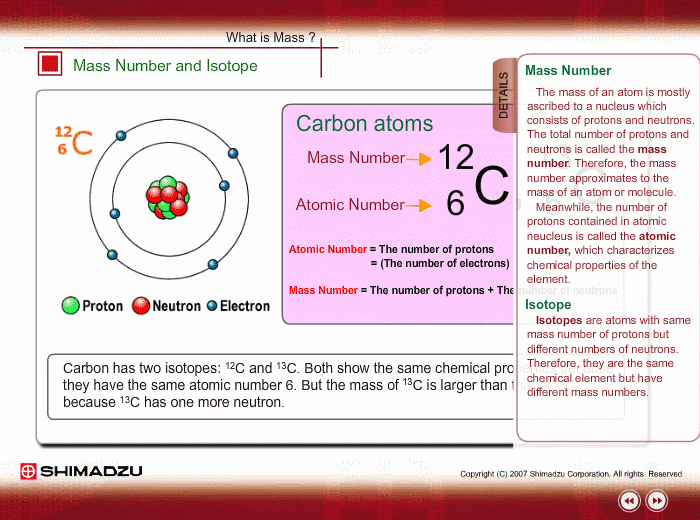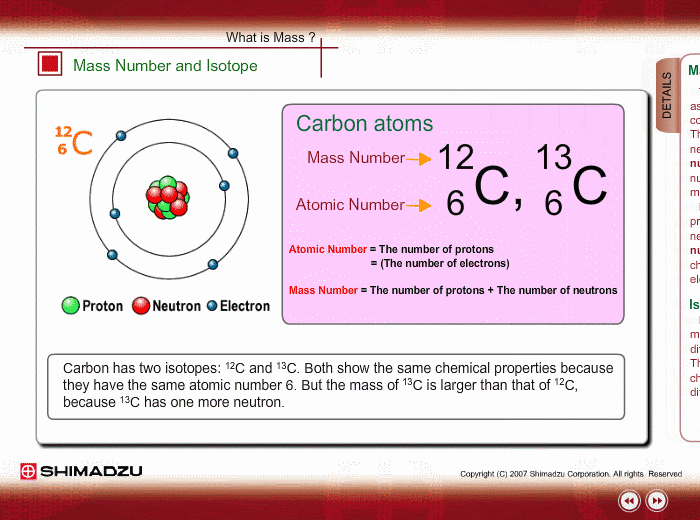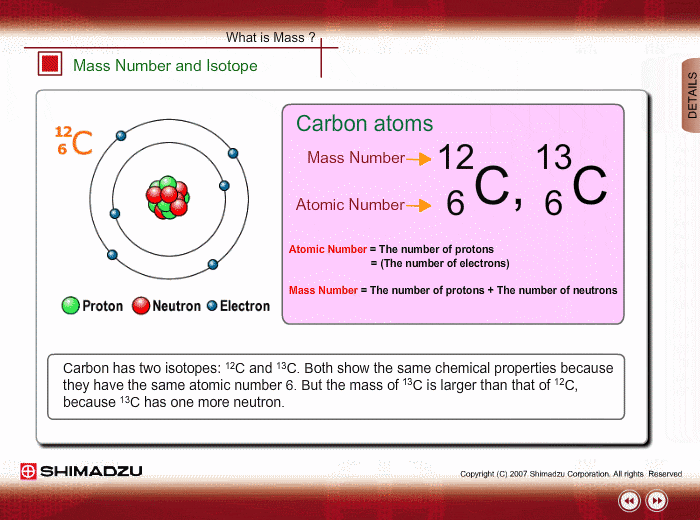
Mass Number And Isotope Shimadzu Shimadzu Corporation

Atomic Structure Of Carbon Atomic Structure Carbon Atom Model Atom Model

Average Atomic Mass Easy Science 10th Grade Science Easy Science Molar Mass

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets

Comments
Post a Comment